Federal and state regulations around veterinary controlled substances are shifting. Here’s what you need to know for 2026.
What’s Changing in 2025-2026
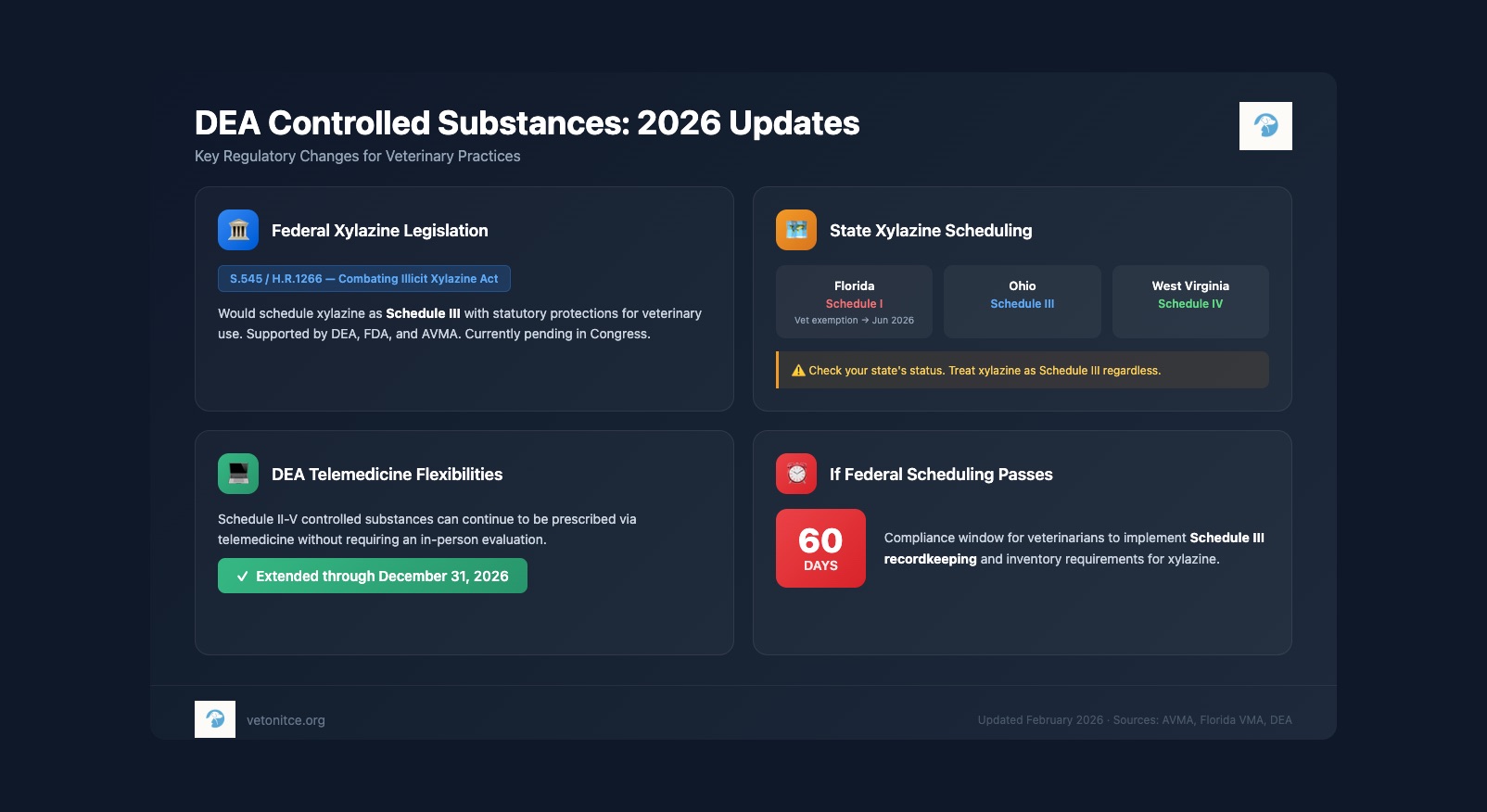
Xylazine: Federal Legislation Pending
The Combating Illicit Xylazine Act (S.545/H.R.1266) was reintroduced in Congress in February 2025. The bill would:
- Schedule xylazine as a Schedule III controlled substance
- Preserve veterinary access through statutory protections
- Allow the DEA to track legitimate supply while addressing illicit use
Both the DEA and FDA support this legislation. The AVMA has called the bill essential, noting that DEA administrative scheduling without these statutory changes could limit veterinary use and risk supply disruption.
If federal scheduling occurs: Veterinarians will have approximately 60 days to comply with Schedule III recordkeeping and inventory requirements.
State-Level Developments
Several states have enacted their own xylazine scheduling:
- Florida: As of November 23, 2025, xylazine is temporarily classified as Schedule 1 with an exemption for veterinary use through June 2026. The Florida VMA recommends treating, storing, and handling xylazine the same as Schedule III substances.
- Ohio: Schedule III
- West Virginia: Schedule IV
This patchwork of state regulations creates compliance challenges for multi-state practices and may affect supply chains.
DEA Telemedicine Flexibilities Extended
The DEA has extended telemedicine flexibilities for prescribing Schedule II-V controlled substances through December 31, 2026. This allows DEA-registered practitioners to continue prescribing controlled medications via telemedicine without requiring an in-person evaluation.
Key Takeaways
- Monitor xylazine legislation at both federal and state levels
- Treat xylazine as Schedule III regardless of your state’s current classification
- If federal scheduling passes, prepare for 60-day compliance window
- Telemedicine prescribing flexibilities continue through end of 2026
Official Sources
- AVMA Xylazine Advocacy: AVMA Position on Xylazine Scheduling
- DEA Telemedicine: DEA Telemedicine Prescribing Rules
- Congress.gov: S.545 - Combating Illicit Xylazine Act
For a complete overview of DEA compliance, recordkeeping requirements, and diversion prevention, watch Dr. Lauren Forsythe, PharmD’s controlled substances lecture in our on-demand CE library.
